Fully-Human BCMA CAR-T Therapy in Beijing China

Table of Content
FUCASO: The Revolutionary Fully-Human BCMA CAR-T Therapy with Unmatched Efficacy and Safety
 FUCASO, developed by Nanjing IASO Biotechnology, is a groundbreaking CAR-T therapy specifically designed to target B-cell maturation antigen (BCMA). This therapy stands out for its unparalleled clinical efficacy and safety profile, providing a new hope for patients with relapsed or refractory multiple myeloma (r/rMM). Approved by the National Medical Products Administration (NMPA) on June 30, 2023, FUCASO has already demonstrated significant success in clinical applications. CAR-T cell therapy by Bioocus International Medical Center is a groundbreaking treatment for certain types of cancer, offering new hope to patients who haven't responded to traditional therapies.
FUCASO, developed by Nanjing IASO Biotechnology, is a groundbreaking CAR-T therapy specifically designed to target B-cell maturation antigen (BCMA). This therapy stands out for its unparalleled clinical efficacy and safety profile, providing a new hope for patients with relapsed or refractory multiple myeloma (r/rMM). Approved by the National Medical Products Administration (NMPA) on June 30, 2023, FUCASO has already demonstrated significant success in clinical applications. CAR-T cell therapy by Bioocus International Medical Center is a groundbreaking treatment for certain types of cancer, offering new hope to patients who haven't responded to traditional therapies.
Cost of FUCASO CAR-T Cell Therapy in Beijing, China
You can get FUCASO CAR-T Cell Therapy Package in Beijing, China by Bioocus International Medical Center with the price starts at $300,000. Please refer to our price list table below:
|
Location |
Cost in USD |
|
Beijing, China |
$300,000 |
Note: price may change and vary depends on complexity of procedures and patient conditions. Click FREE QUOTE button below to learn more.
Advantages of Choosing FUCASO CAR-T Cell Therapy in Beijing, China
-
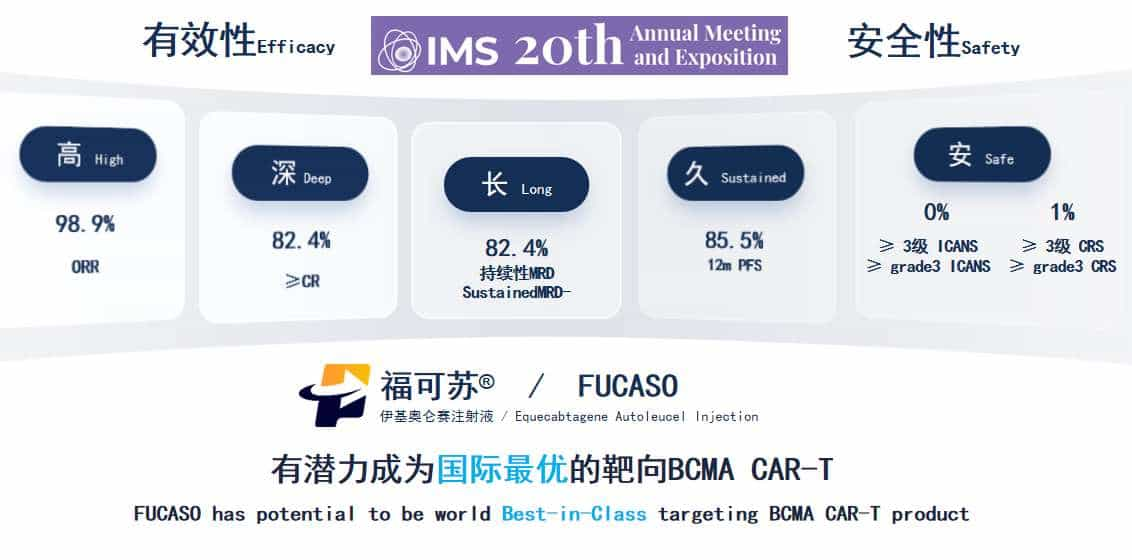
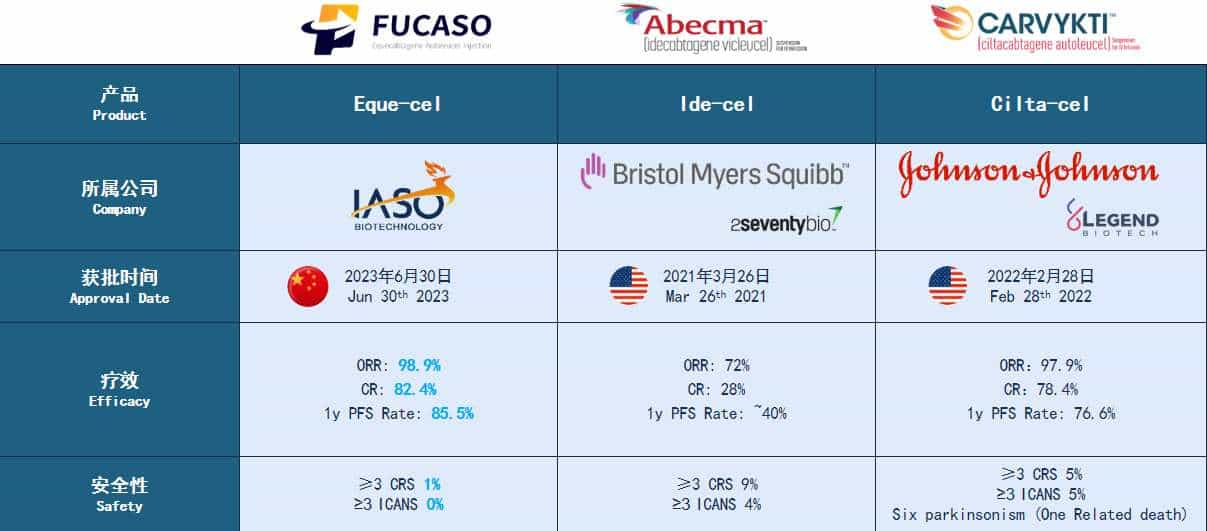
High Response Rates: FUCASO boasts an Overall Response Rate (ORR) of 98.9% and a Complete Response (CR) rate of 82.4%, significantly higher than other available treatments.
-
Durable Remission: Patients treated with FUCASO have shown a 12-month Progression-Free Survival (PFS) rate of 85.5%, indicating long-term remission and improved quality of life.
-
Safety Profile: With ≥3 grade Cytokine Release Syndrome (CRS) occurring in only 1% of patients and no reported cases of ≥3 grade Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), FUCASO offers a safer treatment option compared to other CAR-T therapies
-
Cost-Effectiveness: FUCASO treatment costs significantly less than similar therapies available in the United States, making it a viable option for a broader range of patients.
-
Global Reach: IASO BIO has established a comprehensive treatment center network in China, capable of serving patients from around the world with integrated services from consultation to post-treatment care.
Clinical Success:
The first patient treated with FUCASO has achieved five years of cancer-free survival, a remarkable milestone compared to the median Progression-Free Survival (PFS) of just 2.9 months with standard care. This highlights FUCASO's potential to transform the treatment landscape for multiple myeloma.
Expansion and Future Development:
IASO BIO is committed to expanding the clinical applications of FUCASO beyond multiple myeloma to include autoimmune diseases and other hematologic malignancies. The company is also working on bringing this innovative therapy to global markets, ensuring more patients can benefit from its life-changing potential.
Overview of the FUCASO CAR-T Cell Therapy
The FUCASO CAR-T Cell Therapy procedure begins with the collection of T-cells from the patient’s blood. These T-cells are then genetically modified in a specialized laboratory to produce chimeric antigen receptors (CARs) on their surface. This modification enables the T-cells to recognize and attack specific cancer cells. After modification, the CAR-T cells are multiplied in the lab and then reintroduced into the patient’s body through infusion.
Once inside the body, the CAR-T cells circulate and seek out cancer cells, binding to them and initiating their destruction. This process can take several weeks, during which the patient is closely monitored. The therapy can lead to rapid and significant reductions in tumor size, and in many cases, complete remission. However, the process is complex and requires careful management by a team of specialized doctors and support staff.
FUCASO CAR-T Cell Therapy Package Inclusions
-
Initial Consultation and Assessment: Comprehensive evaluation by the medical team to determine eligibility and customize the treatment plan.
-
T-Cell Collection: Collection of the patient’s T-cells, which is the first step in the CAR-T therapy process.
-
Genetic Modification and Expansion: Laboratory procedures to modify and multiply the patient’s T-cells to create CAR-T cells.
-
CAR-T Cell Infusion: Reintroduction of the modified T-cells into the patient’s body under controlled medical supervision.
-
Post-Treatment Monitoring: Ongoing evaluation and support to manage any side effects and assess the effectiveness of the therapy.

FUCASO CAR-T Cell Therapy Package Exclusions
-
Travel and Accommodation: Patients are responsible for arranging their own travel to Beijing and accommodation during the treatment period.
-
Additional Medical Tests: Any extra tests not directly related to the CAR-T therapy procedure are not included in the package.
-
Post-Treatment Medications: Medications prescribed after the therapy for managing side effects or other conditions are not covered.
-
Personal Expenses: Costs related to meals, personal items, and other non-medical needs are not included.
-
Extended Hospital Stay: If the patient requires a longer hospital stay than initially planned, additional costs may apply.
FUCASO CAR-T Cell Therapy Pre-Op Tests
-
Complete Blood Count (CBC): To assess the patient’s overall health and ensure they are fit for the procedure.
-
Echocardiogram: To evaluate heart function and ensure the patient can handle the stress of the treatment.
-
Electrocardiogram (ECG): To check for any heart abnormalities that could be impacted by the therapy.
-
Imaging Scans (CT/MRI/PET): To accurately locate the cancer and assess the extent of the disease.
-
Infectious Disease Screening: To ensure the patient does not have any infections that could complicate the procedure.
FUCASO CAR-T Cell Therapy Doctor in Beijing, China
Dr. Daopei Lu
|
|
Is the FUCASO CAR-T Cell Therapy Right for You?
-
Refractory or Relapsed Blood Cancer: Ideal for patients whose cancer has not responded to traditional treatments.
-
Good General Health: Suitable for patients who are otherwise healthy and can tolerate the treatment process.
-
Commitment to Long-Term Follow-Up: Patients must be willing to engage in ongoing monitoring after treatment.
-
Personalized Medicine: Best for those who are looking for a tailored approach to their cancer treatment.
-
Desire for Innovative Treatment: CAR-T therapy is a cutting-edge option for those seeking the latest in cancer care.
What to Expect During the FUCASO CAR-T Cell Therapy in Beijing, China
-
Comprehensive Evaluation: A thorough assessment by a multidisciplinary team to determine the best approach for your specific condition.
-
Advanced Laboratory Work: Cutting-edge technology used to genetically modify your T-cells for optimal treatment outcomes.
-
Personalized Care Plans: Tailored treatment protocols designed to address your unique medical needs and goals.
-
Close Monitoring: Continuous supervision by medical professionals to ensure your safety and the effectiveness of the therapy.
-
Holistic Support Services: Access to additional services such as counseling, dietary guidance, and wellness programs to support your overall health during treatment.

FAQs (Frequently Asked Questions)
What types of cancer can CAR-T cell therapy treat?
CAR-T cell therapy is primarily used to treat certain types of blood cancers, such as leukemia and lymphoma.
Is CAR-T cell therapy available for all patients?
Eligibility for CAR-T therapy depends on several factors, including the type and stage of cancer and the patient’s overall health.
How long does the CAR-T cell therapy process take?
The entire process, from T-cell collection to post-treatment monitoring, typically takes several weeks.
Are there any risks associated with CAR-T cell therapy?
While CAR-T therapy can be highly effective, it may also cause side effects, such as cytokine release syndrome, which requires careful management.
Why should I consider CAR-T cell therapy in Beijing, China?
Beijing offers advanced medical facilities, expert doctors, and a comprehensive treatment package that combines quality care with affordability.
Get FREE QUOTE for FUCASO CAR-T Cell Therapy in Beijing, China!
Are you considering CAR-T cell therapy for your cancer treatment? Take the first step towards a healthier future by booking a consultation with Bioocus International Medical Center in Beijing, China through PlacidWay Medical Tourism. Our team is here to guide you through every step of the process, ensuring you receive the best care possible. Contact us today to learn more about how FUCASO CAR-T Cell Therapy can help you achieve lasting remission and improved quality of life.



 At Bioocus International Medical Center, CAR-T cell therapy is expertly led by Dr. Daopei Lu, a distinguished oncologist renowned for his extensive experience in treating hematologic cancers. With over 20 years of dedicated service in the field, Dr. Lu has been instrumental in advancing CAR-T cell therapy, achieving remarkable success in numerous treatments. His profound expertise in immunotherapy and personalized medicine ensures that every patient receives a tailored and cutting-edge approach to their care. Dr. Lu is supported by a highly skilled team of nurses and laboratory technicians, all of whom share his commitment to delivering exceptional results.
At Bioocus International Medical Center, CAR-T cell therapy is expertly led by Dr. Daopei Lu, a distinguished oncologist renowned for his extensive experience in treating hematologic cancers. With over 20 years of dedicated service in the field, Dr. Lu has been instrumental in advancing CAR-T cell therapy, achieving remarkable success in numerous treatments. His profound expertise in immunotherapy and personalized medicine ensures that every patient receives a tailored and cutting-edge approach to their care. Dr. Lu is supported by a highly skilled team of nurses and laboratory technicians, all of whom share his commitment to delivering exceptional results. 








Share this listing