Request a video call
Confirm Your Virtual Consultation
EmCell Clinic Profile Overview

EmCell was the first clinic in the world to use fetal stem cells for treating individuals as a routine procedure and the first in Ukraine to receive a license permitting the application of stem cells. We also hold the world's first patents for the clinical use of fetal stem cells.
Since its opening in 1994, EmCell has gained extensive experience in fetal stem cell therapy and follow-up worldwide. We have performed more than 8,500 transplantations of fetal stem cell suspensions to more than 4,700 patients from 90 countries. EmCell has developed and introduced the largest number of approved and safe clinical protocols into clinical practice.
EmCell's method is very effective, minimally invasive, and has no known side effects. The unique properties of stem cells that we use allow for their application in many diseases, including cases where other methods have proved ineffective. EmCell's in-house procedure for preparing fetal stem cell suspensions, testing and applying them allows us to ensure high-quality transplants and effective treatment. Suspensions containing fetal stem cells are used solely for our patients and are not intended for sale.
EmCell owns the largest bank of fetal stem cell suspensions. This large variety of samples makes it possible for us to find the best solution for each patient.

What are Stem Cells?
Stem cells are precursors of all cell types of organs and tissues in the human body. They play a vital role by ensuring the continuous replacement of aging and dying cells. As we age, however, their number decreases.
Unlike other body cells, stem cells can both maintain an undifferentiated state and turn into specialized cells in response to signals from the organism. Because of their unique properties, stem cells are successfully used in regenerative medicine and in treating various diseases and conditions. Stem cell treatment can result in partial or full restoration of impaired functions, the inhibition of progressive or degenerative diseases, rejuvenation, and a better quality of life for the patient.
There are various sources of stem cells for clinical application: human fetuses, blood from the umbilical cord, bone marrow, adipose tissue, placenta, animals, and so on. Among all clinically applied cell types, we chose fetal stem cells as the core of our treatment. The unique properties of these cells make their use more effective than other cell types.
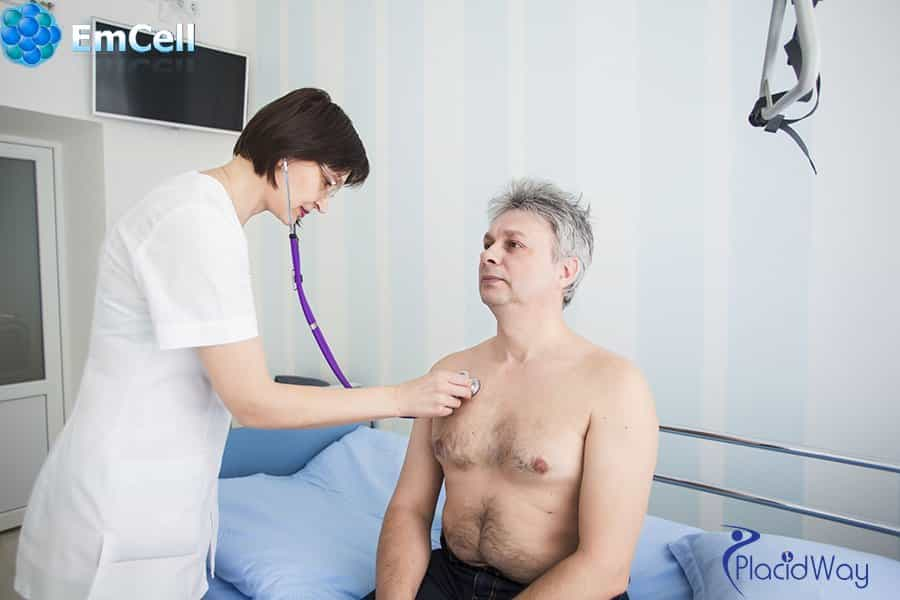
Why Fetal Stem Cells?
-
There is no histocompatibility issue with fetal stem cells. These cells are not rejected by the patient's body as they do not yet contain major histocompatibility complexes. At the same time, cord blood, adult and animal cells express antigens that require complicated procedures such as donor-recipient matching and immune suppression.
-
Fetal stem cells produce a wider range of specialized cells. The ability of fetal stem cells to differentiate is much higher than that of other stem cells, which means that they can produce a larger range of specialized cell types. Fetal stem cell treatment ensures the desired effect and has positive secondary effects on the entire body. This includes the patient's psycho-emotional state, their physical activity, the immune system, homeostasis and the functioning of internal organs.
-
Fetal stem cells have a greater multiplication potential than other stem cell types. This unique property of fetal stem cells allows for them to be applied without prior culturing.
-
Fetal stem cells are not subject to bioengineering prior to transplantation. We use only natural cells. Unlike adult or cord blood-based stem cell technologies, genetic engineering and cloning are not used with fetal stem cells.
-
Fetal stem cell treatment is minimally invasive. Fetal stem cell transplantation differs only slightly from the administration of routine medicines and is performed via drip-feed IV, intramuscularly or subcutaneously.
-
Fetal stem cells have no known side effects. No side effects associated with our treatment have ever been observed.

EmCell Clinic in Kyiv, Ukraine Today
Today, EmCell consists of a Clinical Department and a laboratory complex operating in its own 1,200 sq m renovated building with 8 VIP rooms offering patients comfort and hospitality.
The Clinical Department employs highly qualified, experienced doctors with different specializations who also hold scientific degrees and have numerous patents. Our professional team of nurses and assistants completes our clinical department staff.
The Clinical Department offers both diagnostic (ultrasound, ECG, EchoCG, Doppler, spirography, and so on) and consultative services.
The Laboratory Complex consists of five EmCell lab units functioning as a single department:
-
Biotechnology Laboratory, where fetal stem cell suspensions are produced by original technology developed by our specialists.
-
R&D Department, where our specialists continually work on advancing and improving fetal stem cell suspension quality.
-
Clinical-Diagnostic Laboratory, where lab tests for diagnostic and follow-up purposes are performed and, if necessary, where the treatment regimen is adjusted (complete blood count, blood chemistry, cytology).
-
PCR Laboratory where precise, high-quality regular and express tests take place for both clinical and biotechnological purposes.
-
The Cryobank, where more than 20,000 individual samples of fetal stem cell suspensions, the core of our treatment, are safely stored in a modern cryo-storage facility.

For more information about Stem Cell Therapy or if you need a FREE quote, don't hesitate to get in touch with us!

EmCell Clinic, Kiev,Kyiv, Ukraine Profile Details

History of EmCell Clinic in Kyiv Ukraine
EmCell history is remarkable for inventing fetal stem cell isolation methods and pioneering their practical application.
-
1987 treatment of cytostatic myelodepression and aplastic anemia (filing of patent application)
-
1991 clinical trials and invention of acquired immune deficiency syndrome (AIDS) and HIV-infection treatment method; filing of patent application in the USA (priority dates back to 1993).
-
1992 successful application of fetal stem cells (in vivo) for treatment of type 1 and 2 diabetes mellitus and filing of patent application
-
1993-1994 foundation of the Cell Therapy Clinic of the National Medical University (Kiev, Ukraine). Clinical trials of cell transplants for treatment of different diseases and conditions. First Ukrainian patents were granted. First efforts in development of multiple sclerosis treatment methods.
-
1995 application of fetal stem cells (in vivo) for treatment of amyotrophic lateral sclerosis. First efforts to apply different types of cell suspensions for treatment of muscular dystrophies, in particular Duchenne Muscular Dystrophy

-
1998-2001 Greek, USA, Russian Federation and Ukrainian patents granted.
-
2002 Most Innovative Work Award granted by the Scientific Committee of the Annual Meeting of the Society of Medical Innovations and Technology (SMIT) held in Oslo, Norway. The first Dutch patent granted.
-
2003 the second Dutch patent granted.
-
2004 Ukrainian and Russian Federation patents granted. Development and patenting of methods of intestine diseases treatment (Crohn's disease, non-specific ulcerative colitis, etc.). Working out of ethical norms on the work with human fetal cells approved by the Ministry of Health of Ukraine.
-
2005-2007 extension of active clinical and scientific activities, participation in the numerous conferences and congresses, direct participation in the work of international societies and associations, such as Cellular Therapy Society, Cell Transplant Society, Multinational Association of Supportive Care in Cancer, Society for Minimally Invasive Therapy, EASD.
-
2008 development of methods of fetal cell suspensions application in surgical practice, in particular for treatment of erosive-ulcerative lesions of stomach and duodenum complicated by bleeding. Elaboration of patient surveillance that was declared the best at the 1-st Central European Congress on Surgery (Prague, Czech Republic).

Cell Suspension Bank
EmCell clinic owns modern cell suspension bank equipped with high-technology equipment: modern Dewar flasks and large cryogenic banks (tanks) containing tens of thousands of fetal cell suspensions.
Patents
Patenting has always been regarded as one of EmCell priorities. We were the first to describe the post-transplantation period after fetal stem cell treatment of diseases such as AIDS, HIV-infection, type 1 and 2 diabetes mellitus, aplastic anemia, psoriasis, rheumatoid arthritis, degenerative diseases of the nervous system, inflammatory and cancer bowel diseases, etc. In 1993, International Patent Application Examination Board in the Hague awarded them level "A", or absolutely pioneer works.
In our patents we mentioned the possibility of treating many diseases and conditions caused by or resulting in cell count reduction. Most of all, we take pride in our US and Dutch patents.

We were the first in the world to offer fetal stem cell-based medicinal preparations and mentioned this in our US, Dutch, Russian and Ukrainian patents. We believe that sales of fetal stem cell-based medicinal preparations in specialized pharmacies for domiciliary or hospital treatment will become possible in this century.
This will create the grounds for the development of the new ideology of practical medicine when medical professional will issue prescription for cell composite consisting of HLA-free stem cells. It is they that will help to cope with illness- or age-related cell deficiency.
EmCell clinic welcomes cooperation for the development of this idea.
Scientific Activities
We work in the field of the modern branch of science called cell therapy. And we gave our definition of this branch of science. Cell therapy is science about stem cells and their clinical use.
It was in the 1990s that we realized this was the method's science. If a disease or condition is caused by or results in cell count reduction, cell therapy can be applied.
Almost all severe acquired and genetic diseases cause destruction of actively functioning cells. Stem cells capable of proliferation (multiplication), differentiation (specialization) and morphogenesis (tissue formation) are effective for tissue and organ function and cell count restoration. Stem cells of fetal origin are the most effective in achieving of the above-mentioned effects as they have the highest proliferative potential.

The scope of our scientific interests was wide: it included clinical trials and development of treatment methods for type 1 and 2 diabetes mellitus, AIDS, HIV-infection, leukosis, aplastic anemia, primary and secondary thrombocytopenia, cytostatic myelodepression, cancer diseases, degenerative diseases of central nervous system, multiple sclerosis, amyotrophic lateral sclerosis, muscular dystrophies, such conditions as climax, infertility, aging, as well as functional disturbances of internal organs, chronic fatigue syndrome, application of fetal stem cells in surgical practice, etc.
We regularly attend international and national scientific events where we present our posters, reports and lectures. So far, our professionals took part in a number of international and national conferences and congresses.
EmCell professionals are authors of more than 300 scientific works published in the national as well as international magazines and congress proceedings.
Our clinic owns the trademark EmCell registered in the USA, Russia and Ukraine.

Safety Issues
We are concerned about the safety of the suspensions we use and want our patients feel safe about the treatments offered. We guarantee safety, the system of screening has been developed, which includes the following:
The donor is screened for viruses prior to the procedure.
When collected, stem cells are screened for viruses by our laboratory. Defective and non-viable cells are discarded at once.
After this, suspensions are taken to the certified state laboratory that performs final screening and issues safety certificates for each suspension.
Very often, people are worried about cancer issues. Unlike embryonic stem cells of early development stage (1-2 weeks old, in vitro) characterized by uncontrolled growth and capable of causing teratomas and other complications, stem cells we use have already passed the first stage of specialization, lost the capacity for uncontrolled growth and "know" what cells or tissues to differentiate into. This means that there is no risk of cancer. In 17 years, no post-treatment cancer cases were reported by any patient.
For more information about Stem Cell Therapy or if you need a FREE quote, please contact us!

Location
Reviews
-

Anonymous
Scars are always telling a story. For some they are a reason to brag about an adventure but for others they are a constant reminder of a very painful memory. For men, scars are showing their bravery but a woman is embarrassed and has a low self-esteem everytime is looking at her scars.
Dec 12 2021
-

Anonymous
Dec 02 2021
I am the son of the patient and my name is H.H. My mother is better than before!
-

Anonymous
A success story of a patient who took stem cell treatment for brain injury from Emcell Clinic in Ukraine.
Dec 01 2021

About Medical Center
- Speciality: Alternative Medicine, Anti Aging, Cancer Treatment, Chronic Diseases, ENT, Gynecology Treatment, Infertility/IVF, Neurology, Organ Transplant, Spine Care/Surgery, Stem Cell Therapy, Urology
- Location: 40a Metrolohichna street, Kyiv 03143, Ukraine, Kiev,Kyiv 04073, Ukraine
- Overview: EmCell Clinic in Kyiv Ukraine is the world's pioneer clinic using fetal stem cell transplantation to successfully treat various diseases and conditions, including anti-aging treatment.













.png)






Share this listing